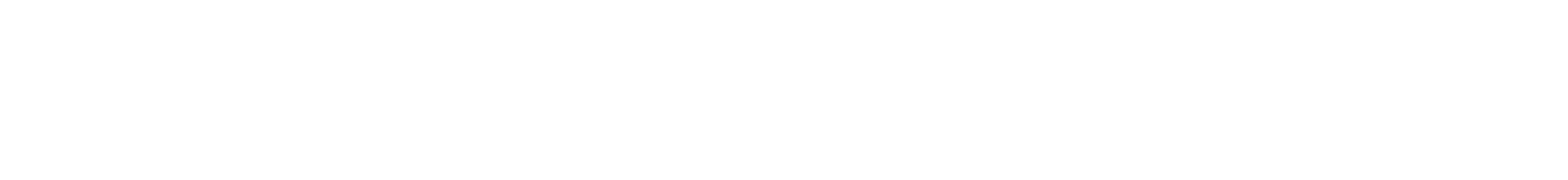On a summer day in 2013, Bob Fontaine and his wife, Ann, were entertaining friends on the back deck of their Cape Cod home when the phone rang. His local doctor was calling with news that the biopsy of a small mole on Bob’s knee showed Stage IV metastatic melanoma.
The following week, surgeons removed as much of the cancerous tissue as possible. But the cancer had spread from his leg to his lymph nodes. Doctors gave Fontaine a 40 percent chance of surviving beyond a year, and that was with the best available treatment option. As a prostate cancer survivor, Fontaine knew he had a battle on his hands.
“Forty percent wasn’t good enough,” says Fontaine, who read everything he could about new advances and experimental treatments. Months of research led him to immunotherapy, and to the Dana-Farber/Brigham and Women’s Cancer Center (DF/BWCC).

Stunning results—for some
Immunotherapy is a type of biologic therapy that boosts the body’s natural defenses to treat and prevent disease. Immunotherapies for cancer provoke the immune system to go after tumors, and program it to continue detecting and clearing cancer cells. The strategy differs in many ways from radiation, chemotherapy, and tumor-removal surgery, which successfully treat many cancers but may miss remnant malignant cells that spread later and can cause serious side effects by damaging healthy cells.
“The idea of using the immune system to treat cancer has been around for a long time, but only in the last decade have many therapies been developed, tested, and proven to work,” says Jonathan Schoenfeld, MD, MPH, director of melanoma radiation oncology at Brigham and Women’s Hospital (BWH), who treats melanoma and cancers of the head and neck.
Immunotherapy has changed the way we treat a number of cancers—starting with metastatic melanoma, for which patients had no treatment options before that improved survival, but now are responding and surviving for many years.
Jon Schoenfeld, MD, MPH
By late 2014, Fontaine’s melanoma was spreading aggressively and time was running out. His oncologist, F. Stephen Hodi, MD, director of the Center for Immuno-Oncology at DF/BWCC, referred Fontaine to an active clinical trial at a hospital in New York City, where enrolled patients received ipilimumab, an experimental immunotherapy drug early studies had shown improved survival for advanced melanoma [the drug was approved for clinical use in the U.S. in 2015].
Fontaine was accepted into the trial. Three months in, his biopsies found no trace of cancer. The trial team, Hodi, and Fontaine’s new dermatologist at BWH, Niroshana Anandasabapathy, MD, PhD, had rarely seen such progress, so fast.
Unfortunately, Fontaine’s story is more the exception than the rule.
“For cancers in which immunotherapy works, patients might respond very well, and are possibly even cured, but this happens for only 20 to 40 percent of people,” says Vijay Kuchroo, DVM, PhD, founding director of the Evergrande Center for Immunologic Diseases. “In our flagship project on colorectal cancer, the Evergrande Center is addressing this question: why don’t all patients, especially with the same type of cancer, respond to the immunotherapy?”

Mixed signals
“Most people think of tumors as being the culprit in cancer,” says Kuchroo. “When you look closer you see it’s not just the tumor, but the immune system is in disarray.”
Most people think of tumors as being the culprit in cancer. When you look closer you see it’s not just the tumor, but the immune system is in disarray.
Vijay Kuchroo, DVM, PhD
Anandasabapathy, a physician-scientist who studies the immune response to cancer, sees the immune system as operating in a constant existential crisis. “It is always asking, ‘What is self, and what is not self?’” she says. “It is constantly trying to balance when to keep the peace with the body’s own tissues and when to go into assault mode against pathogens such as viruses and bacteria. Since cancer is abnormal self-tissue, it’s hard to decide what is the correct response.”
As a clinician and researcher, Anandasabapathy has spent her career investigating why the immune system isn’t triggered by the presence of cancer cells—and more specifically, why the high rate of mutations are not detected by T cells, which are able to successfully identify and clear infections. Her laboratory works to overcome these failures.
“There’s evidence that the immune system sees cancer [in healthy people] all the time and handles it without leaving a trace,” she says. “Yet when the immune system is compromised, as is the case for patients taking anti-organ rejection medications after undergoing transplant surgery, we see a 40-fold increased risk for certain types of skin cancer. We dermatologists encounter a lot of clinical phenomena that shape the way we understand the relationship between the immune system and cancer. That is why for years, we have been treating skin cancers with a number of agents designed to stimulate an immune response.”
BWH pathologist Scott Rodig, MD, PhD, has also been studying immunotherapy for years. He has worked with virtually every cancer specialty group at BWH and DF/BWCC, and searched through thousands of tumor samples to decipher how they dupe the immune system into letting them sneak by undetected.
“Even some patients who respond well to immunotherapy at first can later develop a resistance to it,” Rodig says. “We need to know what the tumor does that causes the treatment to fail later on.”
The truth about how cancer takes hold lies somewhere between a chaotic immune system and tricky tumor behavior. Immunotherapy works in the gray area between, correcting misleading signals between immune cells that interact with the tumors, like T cells.
Anandasabapathy explains T cells take their instructions from dendritic cells, immune cells that devour foreign materials and alert T cells if they caught a pathogen that needs to be destroyed. Looking closely at melanoma tumors, she was surprised to see dendritic cells were uniquely programmed to prevent attacks on what they saw as self-tissue.
“We wanted to understand how and why these dendritic cells are held back or restrained by this program, making their instruction of T cells incomplete to act on the threat,” says Anandasabapathy. “It turns out, when those dendritic cells move into the skin, they share a genetic program—a code that helps them dampen the immune response of T cells to prevent autoimmune disease.”
In their recently published study in Cell, Anandasabapathy and her research colleagues examined the dendritic cells’ code and detected a suppressor of a signaling gene called cytokine signaling 2 (SOCS2). When they turned this gene off in a mouse model, they saw the immune system could effectively detect and reject melanoma and thymus cancer.
“This sheds new light on how the immune system is programmed to see cancers,” she says. “Now we can start developing more precise immunotherapies that go after that program.”
System override
The immune system’s balancing act between keeping the peace and going on the attack is controlled by molecules known as immune checkpoints. Functioning like a security badge, an immune checkpoint can help T cells recognize normal cells as safe to keep around. The trouble is, these checkpoints can also disguise cancer cells as safe and prevent the T cells from destroying them.
Arlene Sharpe, MD, PhD, who with Kuchroo co-directs the Evergrande Center for Immunologic Diseases, was among the first scientists to discover and describe how cancer cells can disguise themselves by using immune checkpoints to escape attack from the body’s immune system. Sharpe contributed to the discovery of some of these proteins, called CTLA-4 and PD-1/PD-L1, leading to the development of checkpoint inhibitor therapies (including ipilimumab, the therapy Fontaine used) that enable the immune system to see through cancer’s disguise, so the body’s immune cells recognize cancer as dangerous and kill it.
“The discovery of PD-1 and CTLA4 checkpoints in the last 15 years has led us and others to ask if there are other ways tumors inhibit immune responses,” says Sharpe. “We know now that the same inhibitory responses that protect us from autoimmunity can turn the tables and stop the immune system from fighting cancer. Clearly there are multiple layers of immunosuppression and we can use this information to identify targets for immunotherapy.”
Sometimes, turning the tables can have positive consequences. Howard Weiner, MD, co-director of the Ann Romney Center for Neurologic Diseases, has been working to solve the pathology underlying multiple sclerosis, an autoimmune neurologic disease that inflames nerve cells and causes a variety of debilitating symptoms. Weiner’s team developed an antibody to precisely target regulatory T cells and unleash the immune system on multiple sclerosis. They were surprised when the preclinical models proved their antibody worked better at shrinking tumors.
“As a neurologist, I never expected I would be publishing a paper about cancer immunotherapy,” says Weiner. “As my team studied a subpopulation of T cells that are supposed to prevent autoimmune disease, we had an idea: if cancer is the opposite of an autoimmune disease, we could turn our investigations around and think about how to restore the immune system’s ability to prevent cancer’s growth.”
Tip of the iceberg
The immune system’s role in combating cancer is still evolving. Schoenfeld adds that if immunotherapy isn’t effective alone for patients with cancer, it is well worth the effort to combine it with other therapies.
“A lot of current research looks at combining immunotherapy with vaccines, chemotherapy, and radiation,” he says. “Some early studies have shown if you kill cancer cells with radiation, the dying cancer cells can stimulate the immune system and tell it what to recognize. A cancer that might have been hidden before might be recognizable in a way it wasn’t before.”
Schoenfeld is hopeful more immunotherapy targets and more combined therapeutic strategies will yield a tidal wave of choices for patients like Fontaine who so recently had no good options.
“We want to figure out how to use radiation in a way that can stimulate the body to keep preventing the cancer from coming back,” he says. “With more immunotherapies, there are more opportunities to try new things. We’re just seeing the tip of the iceberg.”

Expanding possibilities
Since walking away from the two-year ipilimumab trial this spring without a trace of cancer, Fontaine has regained strength and feels healthier than he has in years. He marvels at his fortune to have been part of the first generation to use this life-saving treatment—and hopes more patients will experience the same incredible result.
“I still cringe when I hear the term ‘cured’, because you never really get past cancer,” Fontaine says. “You have to learn how to go on when you know any lump or bump could be serious.”
Had I made a different choice of doctors or institutions, I would be gone now. I’m hopeful the science and cost of immunotherapy will keep improving so it becomes available and affordable to all who need it.
Bob Fontaine
As success stories like Fontaine’s become more common, Rodig believes evidence will mount that immunotherapy can program the immune system to suppress cancer for life, effectively creating immunity.
“It’s exciting to see the clinical responses of metastatic melanoma patients who start immunotherapy, taper off, and eight or more years later, are completely cured or have minimal controllable disease,” he says. “That means their systems continue to actively fight the cancer. That isn’t possible with chemotherapy alone. The excitement about harnessing the immune system is that it is an ongoing mechanism of cancer surveillance that we hope lasts forever.”
A lab technician holds a Petri dish with a bacteria culture.
(Photo by Andreas Reh/Getty Images)
THE MULTITUDE WITHIN
For every human cell in our bodies, there are up to 10 microbes including bacteria, fungi, and viruses. Known collectively as the microbiota, these tiny, single-celled organisms play a fundamental role in our immune systems. What if the secret to immunotherapy lies in this mysterious multitude?
Lynn Bry, MD, PhD, a Brigham and Women’s Hospital pathologist and director of the Massachusetts Host-Microbiome Center, has been studying microbiota for decades to learn how microbes shape the course of disease.
“Microbes provide micronutrients and other benefits for the body,” Bry says. “They’re also essential for maturing your immune system. I think of microbes as microscopic Swiss army knives within your gut that perform a number of essential functions as you grow and mature. If you don’t have microbes early in life, or you don’t have microbes at all, you’re actually immunodeficient.”
Bry’s research has shown that when select organisms stimulate and mature the immune system they can make it more responsive to vaccines and other immunotherapies. In turn, this allows the host to better defend itself from pathogens and even control cancers and autoimmune disease. The evolution of technology in medicine is beginning to back up the theories Bry and her colleagues have been crystallizing for years.
“Next-generation sequencing, with high-throughput microbiologic methods and computational advancements, have really opened up the field, giving us capacity to broadly look at what organisms are present in an ecosystem and what they do to influence the host,” she says. “So now we can take cultures of organisms from a complex ecosystem to see if they are helping to promote health or trigger some sort of disease or influence a specific disease outcome.”
Bry says the next advances in sequencing technology could prompt deeper and more meaningful studies of the microbiome, including in human clinical trials.
“Soon we may be able to account for the inherent diversity of the microbiota, things like the genetic diversity of patients who have food allergies, who may benefit from different organisms that could be important for triggering the correct response within that patient,” she says.
“It’s early days,” says Bry. “We need to prove this with further studies. Our colonizing microbes clearly perform multiple functions, whether within the gut or in other bodily locations. We are focusing on the bacteria that are important around a particular disease process, and working to develop a molecular understanding of their mechanisms of action, and what we can potentially do to leverage the microbiota therapeutically.”