In 1982, at 17 years old, Hugh Herr was a fearless and well-known rock climber when a blizzard trapped him for four days on Mount Washington in New Hampshire. Hypothermic and hours from death, he was rescued and helicoptered to a nearby hospital where doctors tried to save his severely frostbitten legs—but ultimately, needed to amputate both legs below the knee.
Eager to return to climbing, Herr tried the prosthetic limbs available at the time, but was unimpressed. He focused his attention, and eventually his career, on engineering and testing better-performing prosthetic devices, with the goal of developing models that would behave like natural limbs.
While Herr explored the possibilities of prosthetic devices as director of the Biomechatronics Group at the Massachusetts Institute of Technology (MIT) Media Lab, another expert, plastic surgeon Matthew J. Carty, MD, was exploring possibilities for limb transplants at Brigham and Women’s Hospital (BWH). Carty envisioned a leg transplant surgery that maintains essential nerve connections between the new leg and the brain. He wondered how a transplanted leg would perform compared with the best prosthetic devices.
The sensation of a robotic foot responding to what I am thinking and doing is incredible.
— JIM EWING
“During a standard amputation procedure, the nerve signals that coordinate between the brain and limb are cut off, making it nearly impossible for an amputee using a prosthesis to walk on uneven ground or balance on one leg,” explains Carty, director of the Lower Extremity Transplant Program at BWH and Brigham and Women’s Faulkner Hospital.

When Herr and Carty learned of each other’s efforts, their brainstorming led to a new idea: to redesign amputation surgery and develop a prosthesis that could be controlled by the brain. After several years of significant pre-clinical research at MIT and the New England Organ Bank, the team was ready to pilot a new amputation procedure. If successful, the surgery would preserve essential nerve endings in the amputated limb that could improve the amputee’s mobility, reduce pain, and open the door to testing a new robotic prosthesis.
Herr had the perfect patient in mind: his longtime friend Jim Ewing, who suffered devastating injuries in a rock climbing accident. Ewing had spent two years seeking medical help to ease the agony of his shattered left ankle—with little success.
“I felt like I was trapped in a broken, disabled body,” Ewing says. “I was in such constant pain I was willing to do almost anything to make it stop.”
Ewing met with Carty for an assessment and decided to take a chance with the experimental amputation procedure, which was funded through The Gillian Reny Stepping Strong Center for Trauma Innovation at BWH. Within six weeks of surgery, Ewing was walking with a standard prosthesis and rock climbing again.
“It takes a special kind of person to commit to an experimental medical procedure,” Carty says. “We had done a lot of research, but we didn’t know with certainty this was going to work. In gratitude to Jim’s courageous decision to be the first, we named this procedure the Ewing amputation.”
Ewing has no regrets. “It’s tough being an amputee, but it’s led me down this new path and given me a chance to experience groundbreaking technology,” he says.
Following the surgery, Ewing began testing a prototype foot/ankle developed by Herr’s team in collaboration with Carty. The prosthetic limb was designed to connect to Ewing’s muscles so he could control its movements with his brain and feel sensations similar to a natural limb.
Ewing says, “Within minutes of being connected to the robotic prosthesis, I felt like it was part of my body, what Dr. Herr and Dr. Carty call neurological embodiment. The sensation of a robotic foot responding to what I am thinking and doing is incredible.”
While more testing is needed, Carty and Herr expect a version of the prosthesis will be publicly available within a few years. They are already conducting early-phase research for a similar amputation procedure and robotic prosthetic for arms.
Carty explains, “We’re beginning to say with more confidence that both the procedure and prosthesis could work for a large population. It’s an incredible feeling knowing our work could help redefine the care of amputees and improve quality of life for the nearly 1 million people worldwide who have limb amputations each year.”
Bioengineering feats like the one developed by Carty and Herr don’t happen every day. It typically takes 10 to 12 years to translate an idea into a medical device or therapy that is widely available. As their experience shows, success depends on many factors: understanding the problems, asking bold questions, adapting as challenges arise, collaborating with the right people, maintaining financial support—and being wholly committed.
At BWH, bioengineers, physicians, surgeons, and scientists bring their creativity, perseverance, and scientific chutzpah to collaborate on real-life solutions to a host of medical challenges. Among all the bioengineers featured here, more than a dozen startup companies have launched with hundreds of patents awarded.

MENDING CHILDREN’S HEARTS
In 2014, Pedro del Nido, MD, chief of cardiac surgery at Boston Children’s Hospital, was seeking new answers to an old problem: fixing leaky heart valves in children with congenital heart disease or a related disorder. Typically, surgeons stitch the leaky valve to secure it, but the sutures can burst open or cause the valve to constrict as the heart grows. As a result, these children may need as many as five open-heart surgeries before they reach adulthood.
In recent years, a rigid cardiac ring has been developed to use in adults with leaky heart valves—but its rigidity and small size would stunt children’s natural heart growth, deeming it unviable.
Looking for a new approach, del Nido reached out to Jeff Karp, PhD, a bioengineer at BWH. Through previous work together, del Nido was familiar with a biodegradable medical glue Karp invented and wondered if the Karp Lab’s expertise in biodegradable materials could help his team. Karp asked an instructor in his lab, Yuhan Lee, PhD, to lead the project.
“Together with del Nido’s team, we developed a device inspired by the toy known as a Chinese finger trap,” explains Lee. “It’s shaped like the rigid cardiac ring used in adults, but ours contains a braided sleeve with rods of biodegradable material inside, which enable the ring to stretch over time. As the child’s heart valve grows, the expanding tissue pulls on the ends of the sleeve, and the dissolving rods lengthen the device.” After a couple years fine-tuning the technology, the team reported last fall that the device expanded as expected in pre-clinical tests.
“If everything goes according to plan, we should be able to start clinical trials with patients in a few years,” says Karp.

STICKING IT TO CANCER
Natalie Artzi, PhD, is another BWH bioengineer working to solve problems across multiple disciplines. Almost a decade ago, as a postdoctoral student at MIT, Artzi began investigating the challenges of sutures in gastrointestinal surgeries, which involve leaks and complications in as many as 30 percent of cases for patients with colitis, Crohn’s disease, and colorectal cancer.
“When sutures don’t hold after surgery for tumor removal in patients with colon cancer, the follow-up chemotherapy regimen can be delayed by many weeks while they heal, putting them at higher risk of cancer recurrence,” she says.
Artzi’s solution was an adhesive that could be used by surgeons to seal the site of tumor removal, which would eliminate holes created by sutures. Later, when a close friend of hers was diagnosed with cancer and struggling with the effects of chemotherapy, Artzi wondered if the adhesive could be adapted to treat cancer.
“With traditional chemotherapy, less than 1 percent of the drug reaches the primary tumor as it travels systemically throughout the body,” says Artzi. “The rest accumulates in the liver and the kidneys and kills normal cells including immune cells, leading to many side effects including hair loss, anemia, and fatigue. We thought we could figure out how to attach drugs to the adhesive. The adhesive can be injected immediately after tumor resection and solidify within 30 seconds to deliver cancer-fighting drugs directly to the site.”
Artzi drew on nanotechnology to embed a range of drugs in the adhesive, including antibodies and gene therapies. Through this technology, scientists manipulate submicroscopic particles measuring 1/800th of the thickness of a human hair to engineer tiny devices or develop materials that can enhance products, such as fabrics, batteries, and adhesives.
Now, Artzi’s cancer-fighting adhesive, which has shown success in pre-clinical testing of triple-negative breast cancer and colon cancer, is being tested for brain cancer. BWH neurosurgeon Pier Paolo Peruzzi, MD, PhD, approached Artzi to see if her adhesive could be customized to treat glioblastoma—one of the deadliest types of brain tumors with high rates of cancer recurrence.
Now that we’ve deciphered how to engineer the adhesive to carry multiple anti-cancer drugs, cancer is the biggest focus of my lab. You never know where inspiration will lead you.
— NATALIE ARTZI, PHD
“To better understand how the adhesive would work with brain tumors, I observed one of his surgeries, which was incredibly valuable,” Artzi says. “It helped us modify our materials and identify which therapies to include for brain cancer patients. Now, instead of 1 percent of a drug getting to the tumor, we load the adhesive with the drug directly next to the tumor and can program drug release to prolong its presence. Based on our research, this technology seems much superior to traditional systemic chemotherapy.”
Artzi’s journey typifies the multidisciplinary approach of BWH’s bioengineers.
“Until I saw my friend dealing with the side effects of chemotherapy, our work mostly focused on designing devices for the gastrointestinal tract and for cardiovascular and orthopaedic uses,” says Artzi. “Now that we’ve deciphered how to engineer the adhesive to carry multiple anti-cancer drugs, cancer is the biggest focus of my lab. You never know where inspiration will lead you.”

NANOMEDICINE: TINY SOLUTIONS FOR HUGE BREAKTHROUGHS
Today, nanomedicine is used to tackle a wide variety of diseases. But when Omid Farokhzad, MD, started the Laboratory of Nanomedicine and Biomaterials 14 years ago, only 10 academic papers worldwide cited the term nanomedicine. Each time Farokhzad’s colleagues in BWH’s Department of Anesthesiology asked him what it was about, he replied, “It’s an emerging field bringing the ideas of nanotechnology to medicine. Huge things are going to happen.”
He was right. As of this year, more than 19,000 papers directly refer to nanomedicine, and nanomedicine technologies are used in drug treatments, vaccines, implants, and regenerative medicine.
“At first, it was mostly about engineering at the nanoscale to develop systems for carrying drugs within the body to enhance their safety and efficacy,” says Farokhzad, now the director of BWH’s Center for Nanomedicine. “But we didn’t have a clear understanding of how these technologies interact with the body. Now, we’re studying the nano-bio relationship with the goal of creating more precise, personalized medical treatments that respond to changes at the individual level, such as nanomedicines that release insulin only when someone’s glucose is high.”
Like Artzi, Farokhzad is leading efforts to explore how nanomedicine can be used to create new cancer treatments. He points to a project by his colleague Jinjun Shi, PhD, who believes there’s untapped potential to develop drugs that activate genes capable of suppressing tumors. Typically, these therapies work by turning off the genes that cause cancer cells to multiply. With biologists from Boston Children’s Hospital, Shi’s lab created a nanoparticle platform that does both.
“This is the first time the scientific community has developed the basis for a therapy that could attack cancer from these two angles at the same time,” explains Shi, who recently filed a patent for it. “Our pre-clinical studies show the combined therapy could work well to eradicate tumors and have less toxicity than conventional therapies.”
Nanoparticles are also being investigated to expand treatments for traumatic injuries. Mitch Harris, MD, medical director of The Gillian Reny Stepping Strong Center for Trauma Innovation, approached Farokhzad’s group for help after feeling exasperated by a lack of new options to heal large bone fractures and prevent infection. A brainstorm generated a new idea led by Morteza Mahmoudi, PhD, an instructor in Farokhzad’s lab who is developing regenerative medicine approaches that can accelerate the bone healing process while minimizing the risk of bacterial infection.
“If everything goes well, we can help people with large bone fractures recover faster and reduce the risk of further surgeries,” Mahmoudi says. “This would have a huge impact on patients’ recovery and quality of life.”
RACING AGAINST KIDNEY FAILURE
Joseph Bonventre, MD, PhD, sees great possibilities for bioengineering to offer solutions in a totally different area of medicine: kidney disease.
“People with kidney disease have many cardiovascular complications, and individuals whose kidneys have failed and require dialysis face a 50 percent mortality rate in three years,” says Bonventre, chief of BWH’s Divisions of Renal Medicine and Engineering in Medicine. “It’s a staggering, miserable disease that affects millions worldwide, and we’ve had few meaningful treatment advances for those with kidney failure in the 60 years since kidney transplantation and dialysis came about. These methods can sustain life, but can also cause complications and low quality of life.”
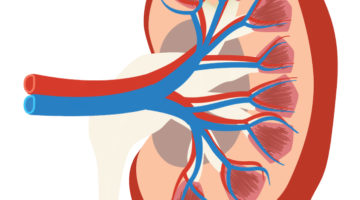
In the search for new treatment options, a research team led by Bonventre made two key advances in 2015 related to growing kidney organoids (miniature kidneys) in lab dishes. First, they found a way to efficiently make these three-dimensional mini-kidneys from stem cells based on patient skin cells, a process that proved challenging to researchers in the past.
The team’s next triumph came when they were the first to successfully model genetic disease in mini-kidneys using stem cell science and a cutting-edge gene editing technique called CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats). For example, when they modified a mini-kidney to carry the gene mutation associated with polycystic kidney disease, a common kidney disease, the lab-dish kidneys formed cysts typical of the condition.
“Our goal is to use these disease organoids to test and bring new therapeutics to patients in a much faster, cost-effective way than what is currently possible through clinical trials,” Bonventre explains.
“These findings are fundamental to our efforts to study kidney developmental abnormalities and many diseases that cause impaired kidney function,” he adds. “The ability to create these kidney structures may one day lead to the replacement of a damaged or diseased kidney with tissue derived from a patient’s own cells.”
Bonventre’s lab is also exploring regenerating kidney tissue to bring back normal function and creating devices that can sustain kidneys, such as a bionic filtration system that could replace the ill-functioning filter structures in diseased kidneys. He is leading a national initiative with the U.S. Food and Drug Administration and the American Society of Nephrology to develop innovative alternatives to dialysis.
“It is fitting that the division that conducted the first successful organ transplant worldwide and played a key role in establishing dialysis technology should now be looking for ways to replace dialysis as we know it,” Bonventre says.
In addition, his team is investigating other approaches to halt the advancement of kidney disease. “We could save an enormous number of lives just by stopping the progression of this disease,” Bonventre says. “It motivates us to continue tackling the problem in new ways.”
BLAZING NEW PATHS
When bioengineers invent new approaches to care, there is rarely a direct path between problem and solution. It takes a lot of brainstorming, experimentation, and vetting to prove an idea can work. When applying for grants to support their work or presenting their ideas to the broader world, sometimes scientists meet with resistance or incredulity.
To this notion, Farokhzad says, “When you’re solving problems in science, beautiful things can happen when you go down a non-linear path not explored before. And that is what’s happening here.”